Ethical issues, ethics committees and research governance
For each survey in the programme, CQC and the respective Survey Coordination Centre seek approval from the Survey Programme ethics committee. Rather than each NHS trust submitting a separate application, the Survey Coordination Centre and CQC submit one application for the survey that covers all participating trusts.
The NPSP ethics committee provide an objective review of applications, providing independent advice on the extent to which proposals for research studies comply with recognised ethical standards. The purpose of this review process is to protect the dignity, rights, safety and wellbeing of all actual or potential research participants. They also seek reassurances regarding issues such as data protection, confidentiality and patient anonymity, and will want to check that proposed research projects will not cause physical or mental harm to patients.
In seeking ethics approval, the Survey Coordination Centre makes a new application for each survey detailing the sampling approach, eligibility criteria and the wider methodology, with associated survey materials such as questionnaires, covering letters and so on, being submitted for review. In being granted ethical approval for a piece of research, it means that the committee has deemed it ‘ethical’. This process can take several weeks but once approval has been given, the Survey Coordination Centre releases the survey materials to contractors and in-house trusts.
Research governance requirements
The Research Governance Framework (2002, 2003, 2005) sets out the principles of good research governance and aims to ensure that health and social care research is conducted to high scientific and ethical standards. It spells out standards and the responsibilities of various parties involved in the research.
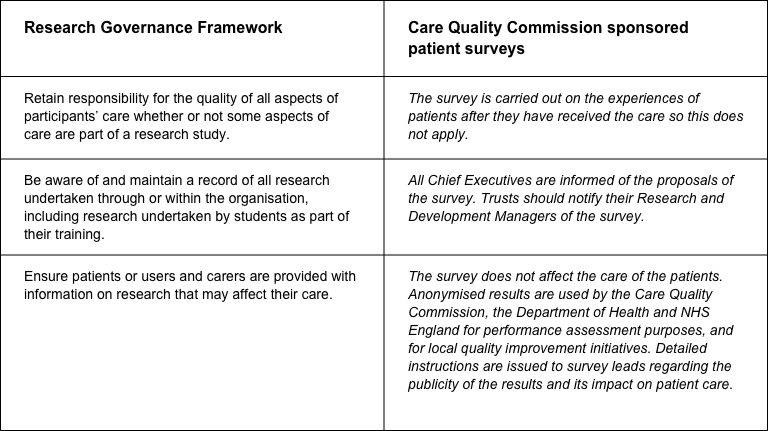
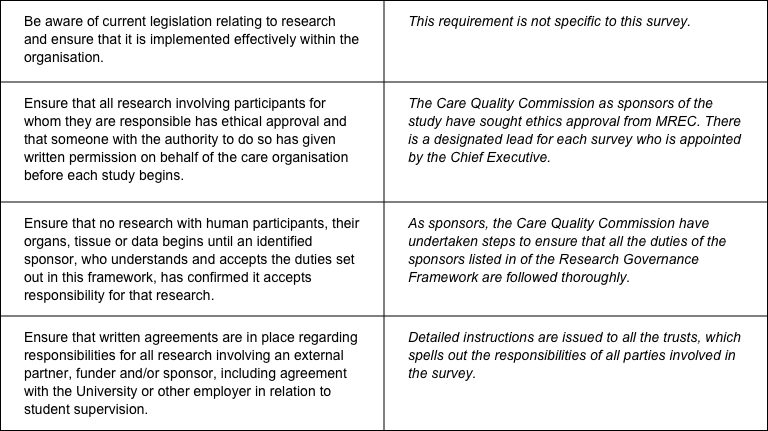
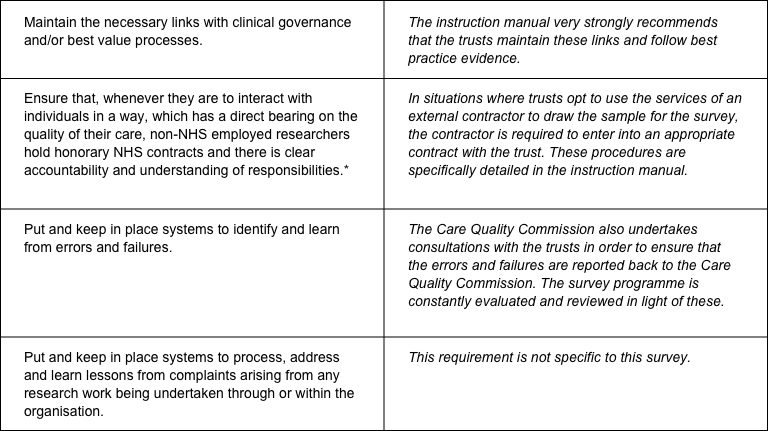
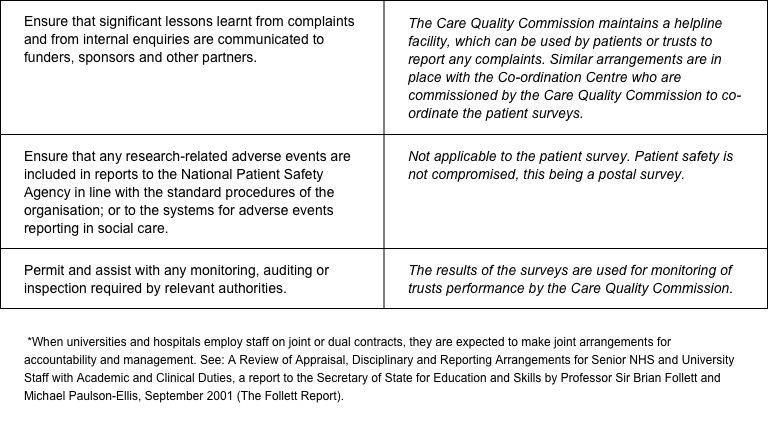
The CQC has produced a table that sets out the responsibilities of organisations providing care and the arrangements made by the CQC for patient surveys.